Aminoleban® EN powder:
The Oral Nutrient for Your Liver
Aminoleban® EN powder is an oral nutritional supplement that contains carbohydrates, fats, vitamins, minerals, amino acids and other elements. It is suitable for people concerned about nutritional deficiencies and liver health1. Provided by Otsuka Pharmaceutical (H.K.) Ltd., Aminoleban® EN powder is an oral nutrient for liver registered with Hong Kong Department of Health. For enquiry, please consult your doctor or pharmacist.

Aminoleban® EN powder:
The Oral Nutrient for Your Liver

Aminoleban® EN powder is an oral nutritional supplement that contains carbohydrates, fats, vitamins, minerals, amino acids and other elements. It is suitable for people concerned about nutritional deficiencies and liver health1. Provided by Otsuka Pharmaceutical (H.K.) Ltd., Aminoleban® EN is an oral nutrient for liver registered with Hong Kong Department of Health. For enquiry, please consult your doctor or pharmacist.
What is Aminoleban® EN powder?
50 g per aluminium package
21’s per Aminoleban® EN powder carton

What is Aminoleban® EN powder?
50 g per aluminium package
21’s per Aminoleban® EN powder carton

Providing branched chain amino acids and micronutrients that the human body needs1. Proteins in the human body are composed of 20 types of amino acids: 9 types of essential amino acids and 11 types of non-essential amino acids. Essential amino acids cannot be synthesised by the human body and need to be taken from diets. Three of the nine essential amino acids are called branched chain amino acids (BCAA), which are leucine, isoleucine, and valine2.


Aminoleban® EN powder has been proven effective in clinical studies to:

Aminoleban® EN powder has been
proven effective in clinical studies to:
Improve serum protein levels3

Improve serum protein levels3

Improve functional performance3

The condition of some patients was improved from Grade 3 or higher to Grade 2 or lower after treatment. This improvement was significant after treatment for 2 weeks, and continued to be so until the end of the 6-month study period.

Improve functional performance3

The condition of some patients was improved from Grade 3 or higher to Grade 2 or lower after treatment. This improvement was significant after treatment for 2 weeks, and continued to be so until the end of the 6-month study period.
Karnofsky performance status can be scored from Grade 0 to 10. Generally speaking, Grade 3 or higher means that the patient’s normal daily life more or less require assistance, while Grade 2 or lower means the patient’s daily life does not require assistance.

Improve quality of life3

Improvement in Karnofsky performance status was significant after treatment for 2 weeks, and continued to be so until the end of the 6-month study period.

Improve quality of life3

Improvement in Karnofsky performance status was significant after treatment for 2 weeks, and continued to be so until the end of the 6-month study period.

How to take Aminoleban® EN powder?1

Ingest with meals three times a day for adults usually
The dosage may be adjusted according to the age of the patient and severity of symptoms
How to take
Aminoleban®
EN powder?1

Ingest with meals three times a day for adults usually

The dosage may be adjusted according to the age of the patient and severity of symptoms
Preparation of Aminoleban® EN powder1
Blender
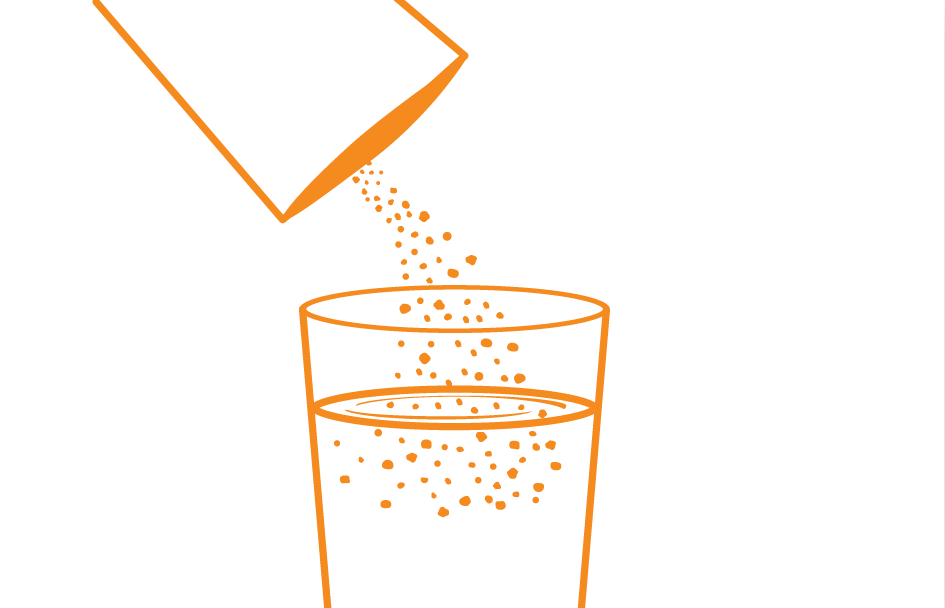
Pour about 180 mL of water or warm water into a blender, add the entire contents of one Aminoleban® EN powder package (50 g) into it.
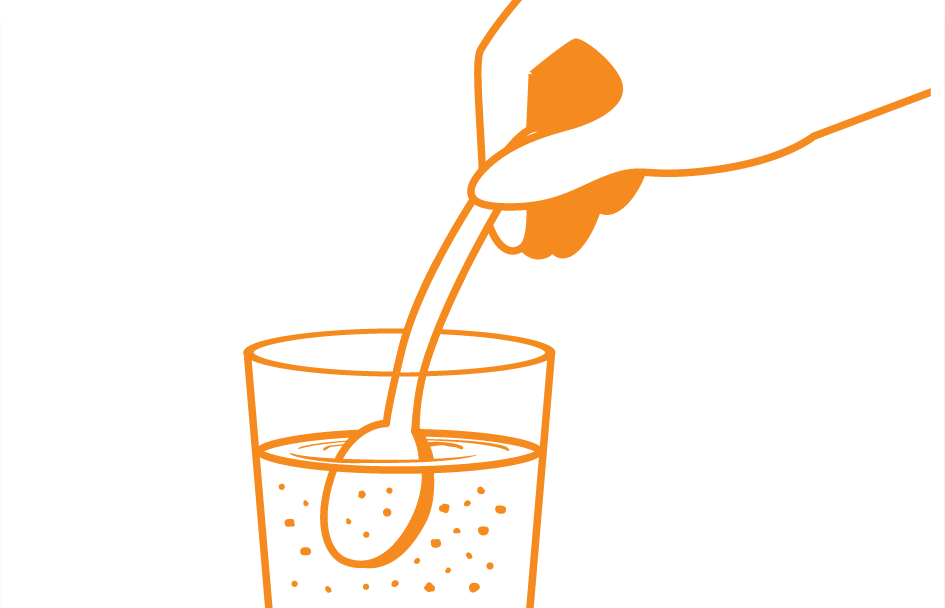
Thoroughly reconstitute
The volume of the reconstitution is approximately 200 mL, and the reconstitution provides about 1 kcal/mL of energy
Aminoleban® EN powder Shaker
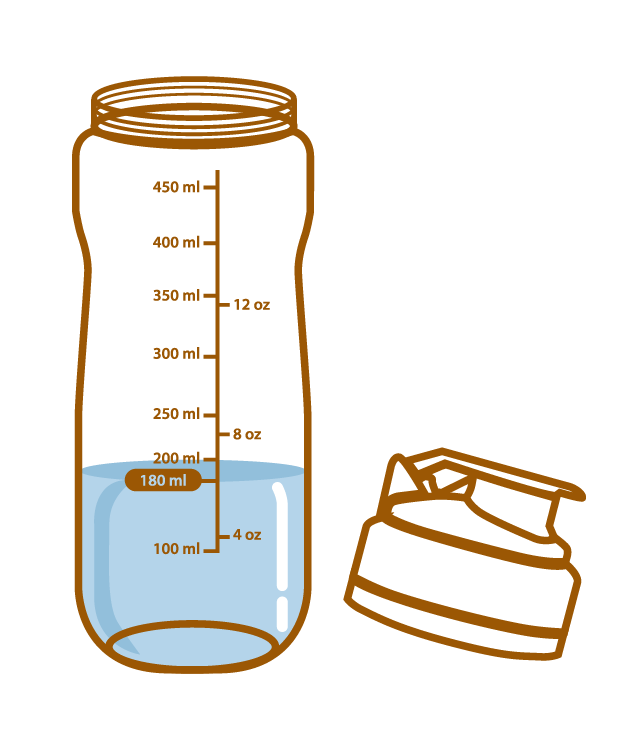
Pour about 180 mL of water or warm water into the Aminoleban® EN powder Shaker
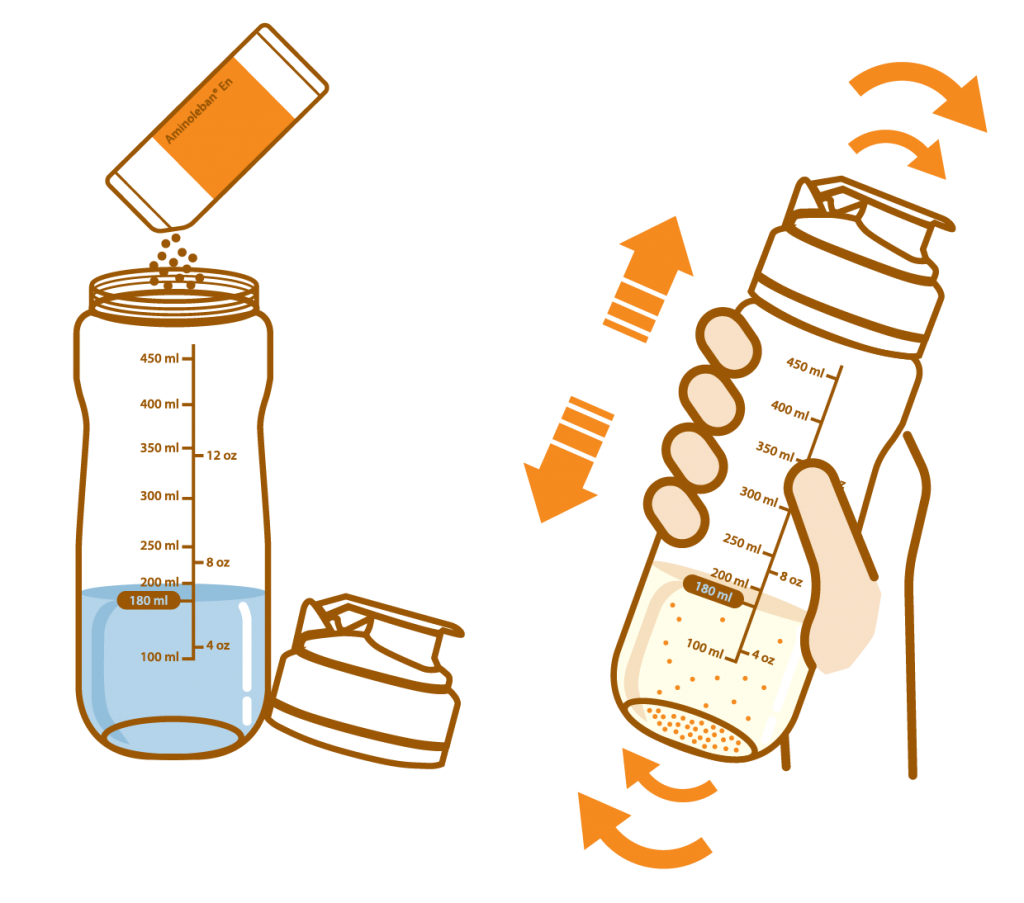
Add the entire contents of one Aminoleban® EN powder package (50 g) into it. Close the lid and thoroughly shake it until the powder dissolves
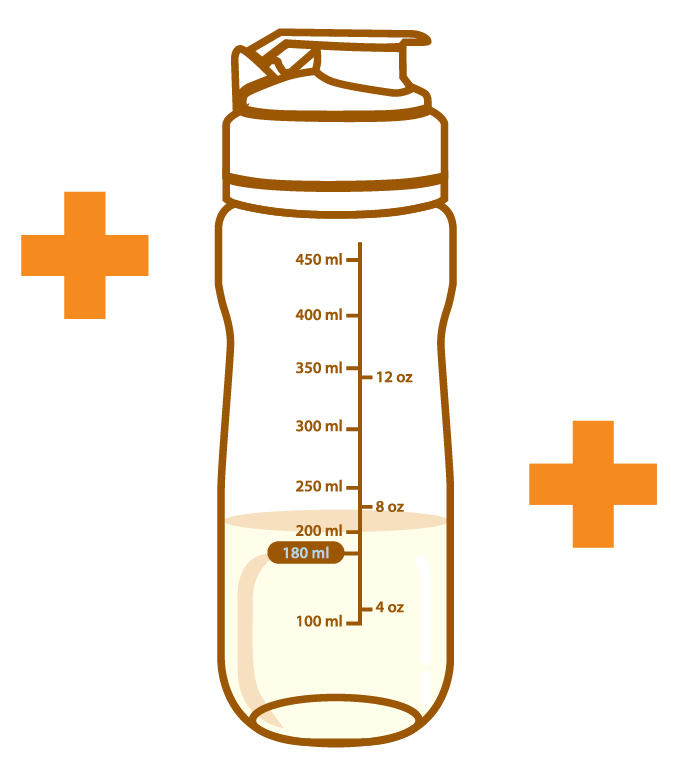
The volume of the reconstitution is approximately 200 mL, and the reconstitution provides about 1 kcal/mL of energy
Preparation of
Aminoleban® EN powder1
Blender

Pour about 180 mL of water or warm water into a blender, add the entire contents of one Aminoleban® EN powder package (50 g) into it.

Thoroughly reconstitute

The volume of the reconstitution is approximately 200 mL, and the reconstitution provides about 1 kcal/mL of energy
Aminoleban® EN powder Shaker
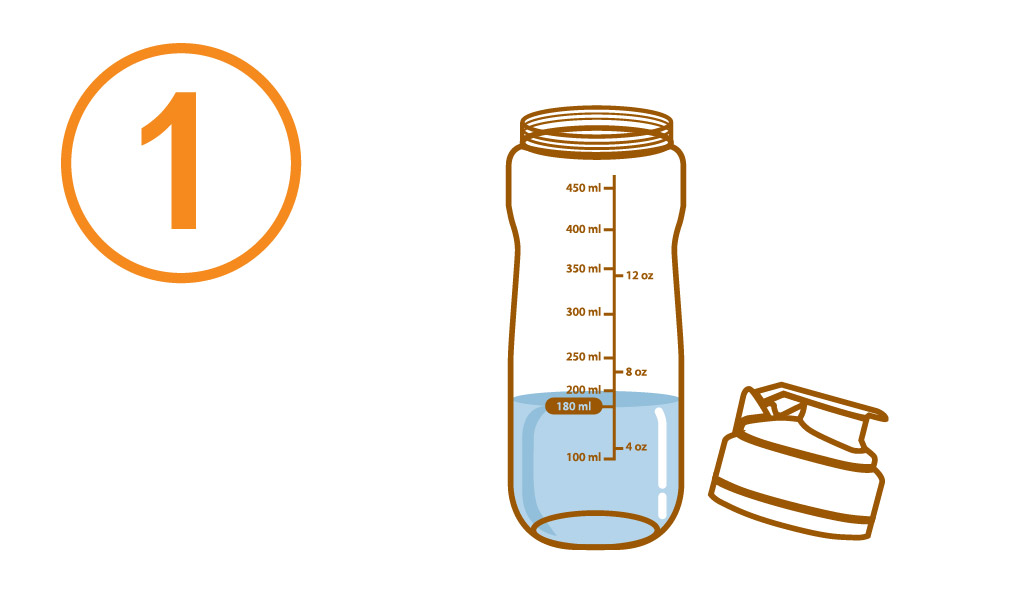
Pour about 180 mL of water or warm water into the Aminoleban® EN powder Shaker
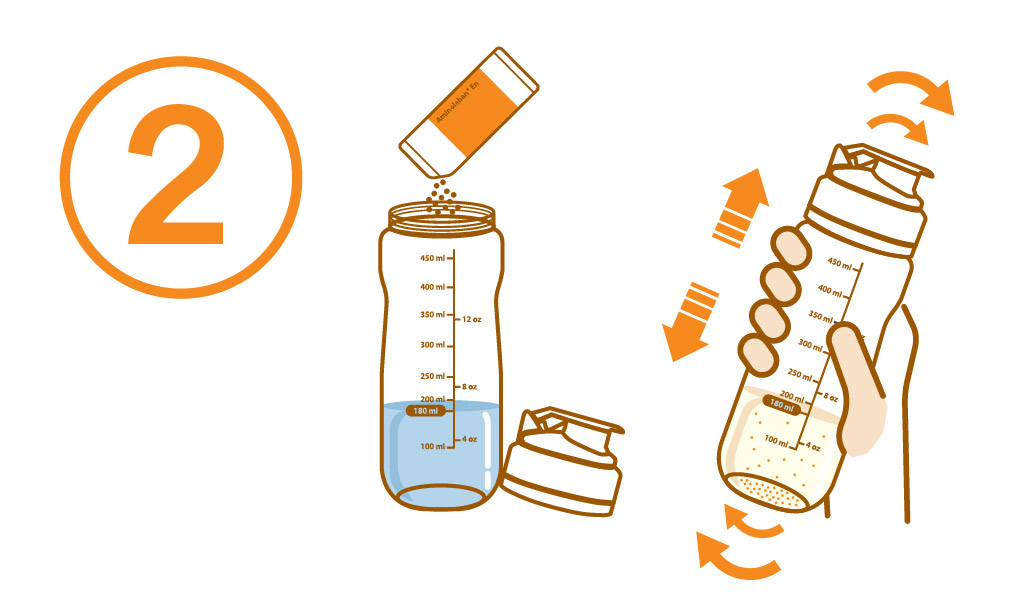
Add the entire contents of one Aminoleban® EN powder package (50 g) into it. Close the lid and thoroughly shake it until the powder dissolves

The volume of the reconstitution is approximately 200 mL, and the reconstitution provides about 1 kcal/mL of energy
Important precautions1
This product is intended for oral feeding and should not be administered into a blood vessel
This product should be reconstituted before use (within 10 hours). If necessary, the preparation should be stored in a cool place
Fibrous vegetables may be mixed with the preparation to improve palatability
Important precautions1
This product is intended for oral feeding and should not be administered into a blood vessel
This product should be reconstituted before use (within 10 hours). If necessary, the preparation should be stored in a cool place
Fibrous vegetables may be mixed with the preparation to improve palatability

Appropriate energy and protein intake
Excessive protein and energy intake is one of the main causes of high blood ammonia levels. On the contrary, insufficient protein and energy intake can lead to nutritional deficiencies. Therefore, balanced and appropriate protein and energy intake is very important for disease prevention


Eating regularly
When the liver has a weaker glycogen storage capacity, three meals a day should be taken regularly with proper nutrition in order to avoid a shortage of liver glycogen


Bedtime snacks
To ensure the body has adequate energy overnight and prevent muscle loss & fatigue, eating light snacks before bedtime (e.g. biscuits, milk and banana, etc) should be considered.
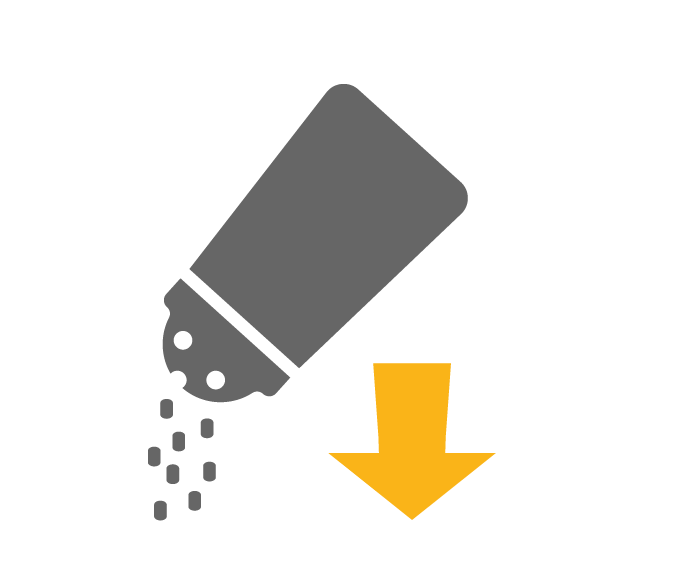

Limiting salt intake
If symptoms such as ascites and oedema are present, the recommended daily salt intake is 5-6 grams


Appropriate energy and protein intake
Excessive protein and energy intake is one of the main causes of high blood ammonia levels. On the contrary, insufficient protein and energy intake can lead to nutritional deficiencies. Therefore, balanced and appropriate protein and energy intake is very important for disease prevention


Eating regularly
When the liver has a weaker glycogen storage capacity, three meals a day should be taken regularly with proper nutrition in order to avoid a shortage of liver glycogen

Bedtime snacks
To ensure the body has adequate energy overnight and prevent muscle loss & fatigue, eating light snacks before bedtime (e.g. biscuits, milk and banana, etc) should be considered.

Limiting salt intake
If symptoms such as ascites and oedema are present, the recommended daily salt intake is 5-6 grams

Frequently Asked Questions1
A:The regular daily dosage (150 g) of this product provides 40.5 g of protein and a total of 639 kcal. When the patient needs more protein and calories, the balance should be supplemented in the patient’s diet.
A:When a patient rejects the product due to its taste (particularly its bitterness), the concentration of the product may be reduced to approximately 0.8 kcal/mL (reconstitute one package [50 g] of the product in approximately 230 mL of water or warm water).
A:When a patient requires restriction of water intake, the dosage of the product may be increased to approximately 2 kcal/mL (reconstitute one package [50 g] of the product in approximately 80 mL of water or warm water).
A:Aminoleban ® EN powder can be taken with most food or drinks. However,
please be reminded that boiling water should not be used for reconstitution to
avoid denaturation of the protein. Fresh fruit juice should not be combined since the acidity of the fruit juices may cause gel formation.
A:Aminoleban® EN powder is generally well tolerated: abdominal distension, nausea, heartburn and abdominal pain occasionally occur; if there is diarrhea, the dose needs to be reduced or stopped. Patients who have allergic reactions to Aminoleban® EN powder, such as rash, itching, should stop taking it.
A:People who have had a history of hypersensitivity to any ingredient of Aminoleban® EN powder should not take it; people who are allergic to milk should also not take Aminoleban® EN powder as it contains casein as an additive. For enquiry, please consult your doctor or pharmacist.
Frequently
Asked Questions1
A:The regular daily dosage (150 g) of this product provides 40.5 g of protein and a total of 639 kcal. When the patient needs more protein and calories, the balance should be supplemented in the patient’s diet.
A:When a patient rejects the product due to its taste (particularly its bitterness), the concentration of the product may be reduced to approximately 0.8 kcal/mL (reconstitute one package [50 g] of the product in approximately 230 mL of water or warm water).
A:Aminoleban ® EN powder can be taken with most food or drinks. However, please be reminded that boiling water should not be used for reconstitution to avoid denaturation of the protein. Fresh fruit juice should not be combined since the acidity of the fruit juices may cause gel formation.
A:Aminoleban® EN powder is generally well tolerated: abdominal distension, nausea, heartburn and abdominal pain occasionally occur; if there is diarrhea, the dose needs to be reduced or stopped. patients who have allergic reactions to Aminoleban® EN powder, such as rash, itching, should stop
taking it.
A:People who have had a history of hypersensitivity to any ingredient of Aminoleban® EN powder should not take it; people who are allergic to milk should also not take Aminoleban® EN powder as it contains casein as an additive. For enquiry, please consult your doctor or pharmacist.
References:
1. Aminoleban® EN Powder Hong Kong prescribing information.
2. Wolfe RR. J Int Soc Sports Nutr 2017;14:30.
3. Ichida F, et al. Acta Hepatologica Japonica 1988;29:1051-61.
4. Yamanashi Prefecture. Points of Diet Management for hepatic patient.
Available from: https://www.pref.yamanashi.jp/kansensho/kansensyou/kannshikkannreshipi/documents/2.pdf (last accessed Nov 2024).
5. NHS Whittington Health. Diet and Liver Cirrhosis. Available from: https://www.whittington.nhs.uk/document.ashx?id=13109 (last accessed Nov 2024).
Aminoleban® EN is a registered trademark of Otsuka Pharmaceutical Co., Ltd. of Japan.
The information on this webpage only includes general information, which is only for the personal reference of those who visit this website, and does not replace the professional advice and instructions given to patients by doctors or pharmacists. For details, please consult your doctor or pharmacist. You should not rely solely on the content contained in this webpage, and we assume no responsibility or consequences for any omissions or errors in the content. Otsuka Pharmaceutical (H.K.) Limited does not endorse or recommend any products disclosed or displayed on this website, and does not make any representations or guarantees regarding any information contained. The information on this website is only applicable to people who visit this website in Hong Kong, and the information contained about the drug may be different from the drug labels of other countries.
Medical professionals should refer to the detailed prescribing information of the product registered with the Pharmacy and Poisons Board of Hong Kong
for complete product information. The information published on this website is not a substitute for your medical judgment. We kindly ask medical staff to evaluate the information provided on this website with professional judgment before making any evaluation or treatment decisions. Under any circumstances, Otsuka Pharmaceutical (H.K.) Limited shall not be liable for any damage, loss or injury caused by using or relying on the information provided on this website in any form.
HKOP-AMN-EN-202412-001

















